
Research
-
Organic reactions are performed by default in organic solvents with molecules of reactant(s) dissolved and moving freely within the isotropic medium. Water was and still is, considered an impediment in conducting organic reactions and obtaining high yields. In stark contrast, in biological systems reactions proceed quickly and efficiently with substrates confined within a crowded cellular environment surrounded by water. Reaction sites are usually situated in the hydrophobic pockets of enzymes, Nature’s catalysts. Enzymatic catalysis is efficient, selective, and enables high yields even with relatively unreactive compounds.
In aqueous media, organic reactions are usually performed in micelles, supramolecular spherical reactors formed by the self-assembly of amphiphilic molecules. Micelles solubilize organic compounds, provide a hydrophobic environment as a reaction site, and increase the local concentration of reactants. Mild reaction conditions involving room temperature reactions and the absence of organic solvents are enticing reasons for the rapid growth of the number of organic reactions performed in micelles.

Research
In aqueous media, organic reactions are usually performed in micelles, supramolecular spherical reactors formed by the self-assembly of amphiphilic molecules. Micelles solubilize organic compounds, provide a hydrophobic environment as a reaction site, and increase the local concentration of reactants. Mild reaction conditions involving room temperature reactions and the absence of organic solvents are enticing reasons for the rapid growth of the number of organic reactions performed in micelles.
However, micelles do not exploit many of the beneficial features of enzymes, such as catalytic activity and selectivity. In contrast, the broad field of artificial enzymes provides solutions for efficient catalysis, but at the same time, there are bottlenecks. The scope of reactions and substrates is limited, usually complex molecules are used, but above all the low solubility of organic molecules in water prevents wide application for organic synthesis. While the prospects of performing organic reactions in water are becoming evident, incorporating enzyme-like catalytic activity into these systems remains an unattainable challenge.
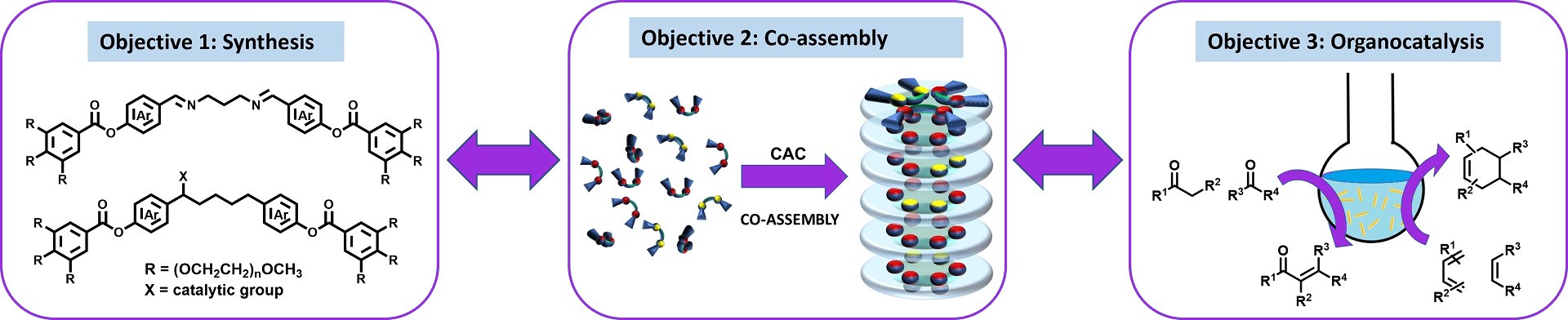
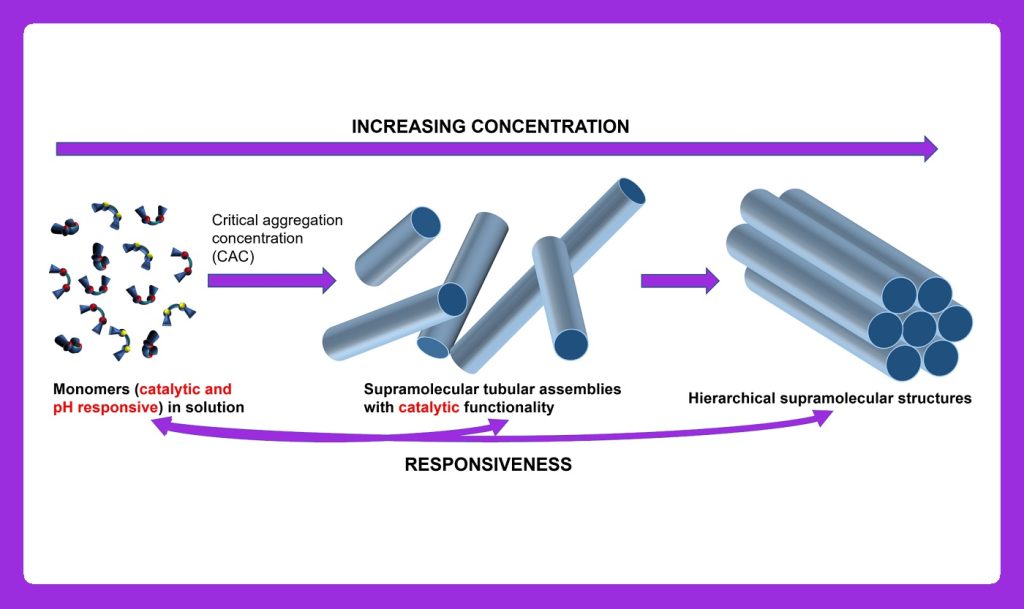
This project aims to develop a pH-responsive system for efficient catalysis of various organic reactions in water in a wide concentration range, from micromolar to millimolar and beyond by implementing self-assembly and supramolecular approach.
In STARTNOW project organic reactions in water will be conducted by employing artificial tubular supramolecular structures assembled from bent-shaped amphiphilic molecules in water. The reaction site is a hydrophobic pocket of the optimal size that holds reactants in a confined space and prevents the formation of side products. The proximity and availability of reaction centers, i.e., organocatalytic moieties, are enabled by the tubular shape of these nanoreactors. Dynamic covalent bonds within the molecular structure ensure the dissociation of molecules upon the small pH change, leading to the disassembly of tubular architectures and the release of products.
For successful management of the project, three highly interrelated and complementary research objectives are devised:
Objective 1
The synthesis of two types of amphiphilic water-soluble molecules (monomers), one with a pH-responsive moiety and the other with organocatalytic functionality
Objective 2
The study of their self-assembly and co-assembly into tubular supramolecular structures in water
Objective 3
Utilizing developed systems for the catalysis of specific organic reactions based on the incorporated organocatalytic monomer.